-
Is fullerene C60 large enough to host a multiply bonded dimetal?
I. Infante, L. Gagliardi and G.E. Scuseria
Journal of the American Chemical Society, 130 (23) (2008), p7459-7465


DOI:10.1021/ja800847j | unige:67 | Abstract | Article HTML | Article PDF
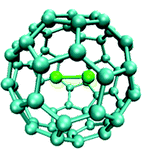
Some dimetal fullerenes M2@C60 (M = Cr, Mo, W) have been studied with computational quantum chemistry methods. The transition metal diatomic molecules Cr2, Mo2, W2 form exohedral complexes with C60, while U2 forms a highly symmetric endohedral compound and it is placed in the center of the C60 cavity. This highly symmetric structure is an artifact due to the small size of the C60 cavity, which constrains U2 at the center. If a larger cavity is used, like C70 or C84, U2 preferentially binds the internal walls of the cavity and the U−U bond no longer exists.